Concept of CAR T cell therapy
Chimeric antigen receptors (CARs) are artificial surface receptors that can be introduced into somatic cells by genetic engineering. They act as recognition molecules like antibodies or T cell receptors. In this respect, CARs are used for cellular therapy to redirect T cells towards killing of cancer cells. In general, the concept comprises the following steps:
- Isolation of T cells from the patient‘s blood using, e.g., MACS® Technology (magnetic cell separation)
- Activation and expansion of isolated T cells
- Transduction of T cells with CAR construct using e.g. lentiviral vectors
- Expansion of CAR T cells and infusion into patient
 © Miltenyi Biotec
© Miltenyi Biotec
Workflow for the manufacturing of genetically modified T cells
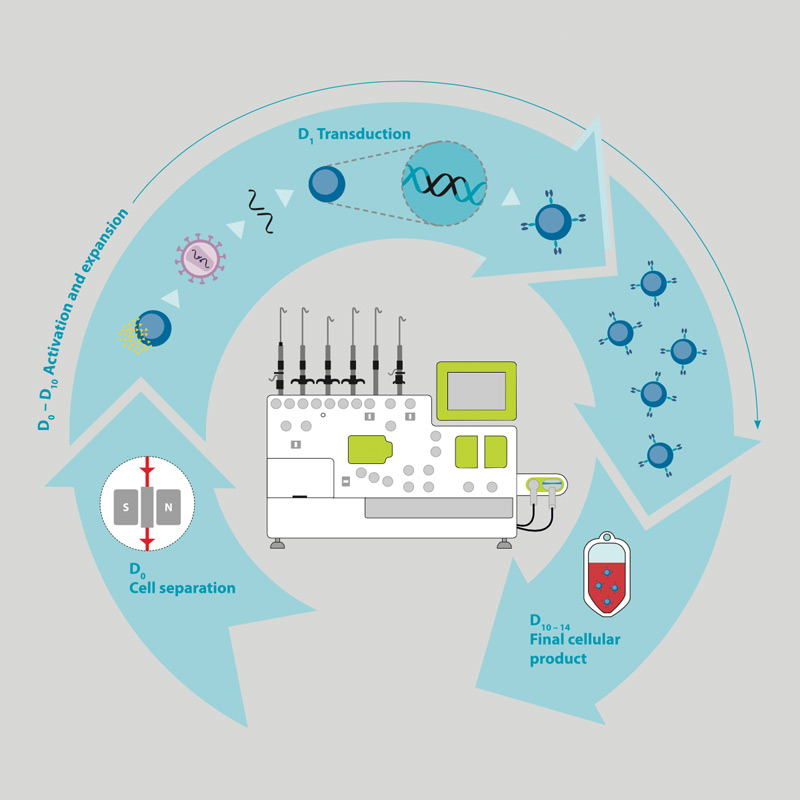
A number of complex individual steps are required for the manufacturing of genetically modified T cells. In order to extract the cells from patient's blood (leukapheresis), T cells are magnetically enriched (cell separation, day 0).
In the activation and expansion phase (day 0–10), the T cells are activated, provided with a CAR molecule (transduction, day 1) by using e.g. lentiviral vectors and proliferated (expansion). CAR-T cells are optionally purified again and then filled in the final formulation solution (cellular end product, day 10–14).
GMP-compliant manufacturing and QC
CAR T cell products for the clinical trial will be manufactured in accordance with GMP guidelines in Ulrike Köhl’s lab at Hannover Medical School (MHH). QC (quality control) and tracking of the cell products will be monitored closely.
- The entire manufacturing process is facilitated by the CliniMACS Prodigy® Device that automatically performs the following consecutive steps 2-6 in a closed system with minimal user interaction.1, 3, 5
- Patient undergoes leukapheresis, blood sample is taken, and leukapheresis product is transported to the manufacturing site at +2–8°C. Delay between blood harvesting and initiation of the process <24 h
- CD4/CD8-positive cells are magnetically enriched on the CliniMACS Prodigy Instrument (day 0).
- CD4/CD8-enriched cells are activated.
- Activated T cells are transduced with the CAR construct using the lentiviral vector (day 1).
- T cells are expanded (day 1 - 12), remaining vector and activation reagent are washed out on day 3.
- Drug substance formulation: CliniMACS Prodigy Instrument washes and resuspends cell product in approx. 100 mL of infusion solution into the target cell bag (day 12).
Manufacturing of cellular product
 © Miltenyi Biotec
© Miltenyi Biotec
Clinical trial
- Leukapheresis of the patient for manufacturing of investigational medicinal product (IMP) (day 14)
- Lymphodepletion: (Cy 60 mg/kg day -7 and -6; Flu 25 mg/m2 day -5 to -1)
- Dosing (“3+3” design): one dose i.v. day 0
- 105 CD20CAR transduced T cells per kg body weight (BW), 3 (+3) patients
- 106 CD20CAR transduced T cells per kg BW, 3 (+3) patients
- 107 CD20CAR transduced T cells per kg BW, 3 (+3) patients - Additionally, up to 6 patients will be treated with highest tolerated dose, resulting in an approx. number of 15 patients.
Intervention scheme of CD20 CAR-TIME trial
 © Miltenyi Biotec
© Miltenyi Biotec
Monitoring and concomitant research
IPC (in process control)/QC (quality control) strategy for the investigational medicinal product (IMP) and its intermediates:
The monitoring of cell products includes essential parameters that are needed for its final release (cellular composition/viability, transduction rate, microbiological safety tests, and quantification of vector copies per cell). Specifications for IPCs and QC testing were defined taking into consideration published experimental data, statistical inference, long-lasting expertise of the manufacturing teams in the QC of various cellular therapeutics, e.g., stem cell products, activated NK cells4, and antigen-specific T cells8, as well as first protocols for the generation of clinical-grade CAR T cells.
Samples of (i) the leukapheresis product, (ii) CD4/CD8-enriched T cell fraction (after magnetic enrichment), (iii) cells during cultivation on day 5 before lymphodepletion of the patient, (iv) the drug substance, and (v) the final drug product will be analyzed.
Concomitant research carried out in the lab of Hinrich Abken at the University Clinic Cologne will investigate the in vivo functionalities of CAR T cells, e.g., their persistence, cytotoxic and proliferative capacity.6
Monitoring and concomitant research protocol
 © Miltenyi Biotec
© Miltenyi Biotec